This activity is provided by Med Learning Group.
This activity is supported by an independent medical education grant from Jazz Pharmaceuticals.
Copyright © 2025 | Med Learning Group | All Rights Reserved | Website by Divigner
This activity is supported by an independent medical education grant from Jazz Pharmaceuticals.
Copyright © 2025 | Med Learning Group | All Rights Reserved | Website by Divigner

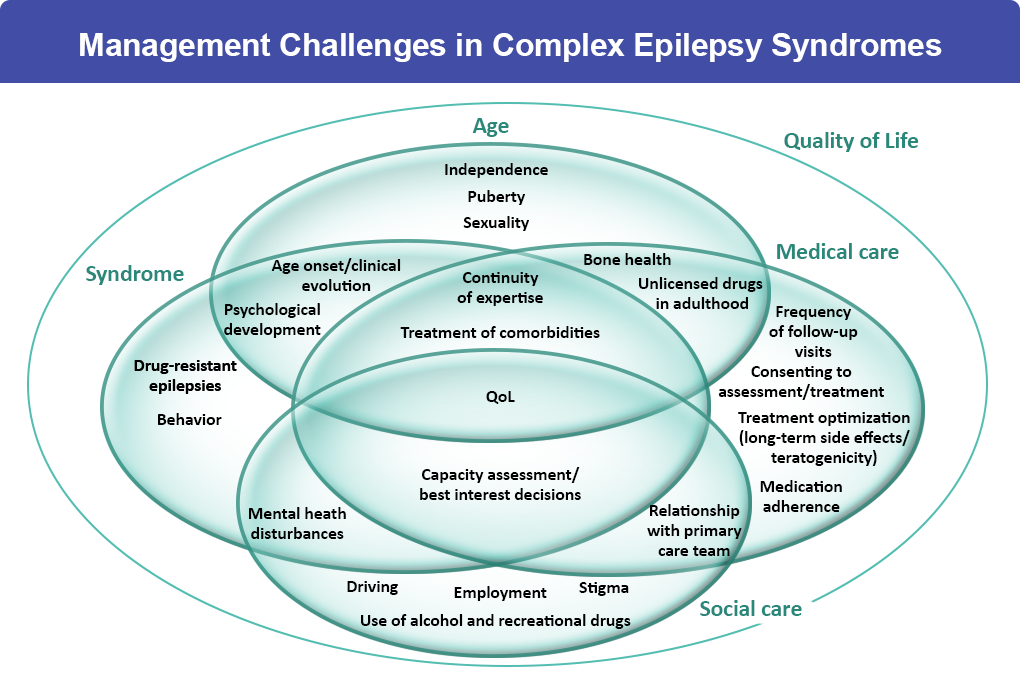
 Accurately diagnosing the cause and specific type of DEE can better help to determine the most appropriate treatment. Treatment and management options for DEEs may include:3
Accurately diagnosing the cause and specific type of DEE can better help to determine the most appropriate treatment. Treatment and management options for DEEs may include:3